Health and welfare
Thursday 01 April 2021 | 23:30
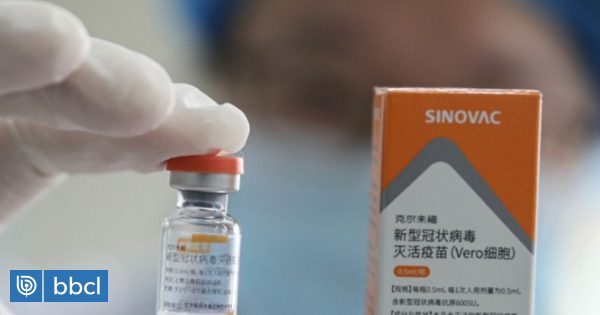
Vaccine experts from the World Health Organization (WHO) said Wednesday that an interim analysis of data from clinical trials of two Chinese anticovid injections showed “safety and good efficacy”, but lacked information.
“Vaccines demonstrated protection and good efficacy against Kovid-19 when the disease presents symptoms, but data are lacking (…) in the elderly and people with other diseases,” experts from the Strategic Advisory Group (SAGE) WHO indicated on the vaccine
Once they are released, efficacy and safety studies on these two vaccines will be required “to evaluate their effects in these groups”.
These experts, who met on 22–25 March, studied vaccines at Chinese laboratories Sinopharm and Synovac.
The WHO’s decisions on homologation applications submitted by both laboratories are expected from early April.
Under an emergency process, WHO approval allows countries to expedite their own regulatory approval processes for the import and administration of vaccines.
Alejandro Creviotto, president of the SAGE group, indicated that experts would wait for the WHO’s decision on the homologue before publishing its recommendations on the use of Chinese vaccines.
On December 31, 2020, the WHO granted its first emergency approval for the Pfizer / BioNTech vaccine, followed by injectables from AstraZeneca and Johnson & Johnson.



